MASSIVE FORCES
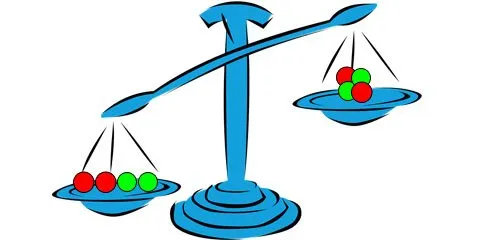
Lets grab an imaginary scale that weights really small things and compare the mass of, for example, two protons and two neutrons by separate vs the mass of the helium nucleus (He), that contains an equivalent amount of protons and neutrons. The result, is this amazing piece of artwork I made in 20 seconds in Photoshop after fetching a pixabay vector:
A Helium nucleus with two protons and two neutrons weights less than the particles weighted by separate.
What's up with this missing mass? The answer lies within an equation that is very, VERY famous (thank you Albert!):
E = mc^2
This is so brief yet it contains a LOT of information! it tells us that energy and mass are not so different after all, that they are equivalents!. And this is so true that it justifies the mass difference in our imaginary scale. When we join partivles to build a He nucleus, a part of its mass transforms into unleashed energy. The formed nucleus is more stable, thanks to the fact that it released energy to pack up. If we want to break it apart, back into its basic elements, we need to resupply that energy that was released, this energy is called "nuclear binding energy"; this is the mass that protons and neutrons lost.
This kind of nuclear reaction, where small things as joined to make larger ones is called fussion (you don't need to be Oppenheimer to figure that out, do you?). There's a trick to it, you've to persuade the ingredients into getting very close to each other so that the attraction forces may interact. And "persuading" in this case implies fighting against and defeating the electrostatic repulsion force. It's hard, but it can be done. The stars; including, of course, our beloved Sun; are excellent examples of fusion reactors. We were also able to emulate that here on Earth, and there's a WIP of a fusion reactor that may create energy in a very efficient way.
BOOM
(On topic audio)
Perfect, bind two light nucleus and get a lighter one, getting energy in the meantime. That way we can "cook" atoms until we hit Iron (Fe). Well, things get a bit uphill for stars at that stage of their lives, since fusing two Iron nucleus COSTS energy. For elements heavier than iron our scale reverses: now the nucleus is heavier than the ingredients we'd use (dammit! we wanted to make gold!). Therefore, energy profit there is only achieved by breaking the nucleus apart. This process is called fission.
By fusion or fission all paths lead to Iron: What happens to an atom (liberating energy by fusing or by breaking apart) depends, roughly, in being heavier or lighter than Iron.
There's different ways of breaking apart an element. A good option is the classic sledgehammer, hit it with something to break it! The problem is that to "hit" something you've to be able to touch it, and that is kinda hard to do with things that have a positive charge. Here is where we call our Champion, the neutron. With no charge, he can reach a nucleus with no problems and puncture it.
When a nucleus absorbs a neutron it may become unstable and it may even break apart... this is what we are interested in. Remember that when it breaks apart it releases energy.
"Wouldn't it be great to find an element that besides releasing energy it'd released another neutron to break another nucleus and then keep on releasing energy?"A question like this is what Leo Szilard, the guy that conceived the idea of triggering a chain reaction, did in 1933, a year after the discovery of the neutron, but when nobody even imagined that fission existed.
By the end of 1938 (7 years before the bomb) it was discovered that Uranium could fission when it was bombarded by neutrons; a few months later, it was found that it released more neutrons when it did. Yet, another turn of the screw was needed to find: only one of the isotopes of Uranium fissioned releasing more neutrons, Uranium-235 (92 protons & 143 neutrons).
The radical difference is because nucleus, just like thugs, onions, some superheroes and electron atomic orbitals, have layers; this is, allowed levels where they place the nucleons (protons & neutrons). And just like in chemistry different amounts of electrons in the same layer grant different chemical behaviors, the fill-up of nucleons around the nucleus has a similar effect in a nuclear perspective. This is why the nucleus have more or less stable states depending on the amount of nucleons they have. This is why some isotopes fission so easily when they are hit by a neutron (like Uranium-235), and others don't (like Uranium-238).
Since we're always three cents short of a dollar, more than 99% of the natural Uranium is in shape of the Uranium-238 isotope, while less than 1% is the one we'd love to fission. There's different ways to palliate the despotism mother nature imposed us, that consider different kinds of nuclear reactors (that perhaps I may talk about in future articles). Long story short: by concentrating Uranium-235 or reducing the neutron loss to a minimum level.
The important part is that Uranium-235 is capable of generating a self sustained chain reaction: A neutron hits the Uranium, breaks it apart and generates more neutrons... that go break more neutrons... and so on.

Source: I've no frikking idea of where did Giphy stole it from.
The amount of generated neutrons is 2 or 3, but not all of them are going to hit another Uranium-235: Some will escape, others will be absorbed by things that do not fission, and other will be seized by customs (joke). Anyways, if we manage to create a system where, in average, a neutron is generated per neutron spent... BOOM! We've got a nuclear reactor.
Now, what happens if we build a system where a lot of Uranium-235, with a structure where very little neutrons may escape? In that case, a fission would generate energy, rounding up, 2 neutrons that would trigger 2 fissions, that would generate 2 neutrons each; 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1024, 2048, 4096, 8192, 16384, 32768, 65536... Anyone that knows how an exponential works, things get ugly very, very fast. Place that system in the corresponding container, add a detonator to trigger the chain reaction, give it a fancy name, throw it from a plane... And you'll get result similar to the first image of the previous part of this article.

(Now, just because a reactor and a bomb work because of the same phenomenon does not imply that a reactor can become a bomb, or vice-versa (yes, Hollywood got it wrong, again). The conditions on both are very different, for example, the concentration of Uranium-235 present in a bomb -the one that fissions- is around 20 times higher than the one of a reactor.
ELEMENTAL, (my dear Watson)
Lets go back to ancient Greece (again), if you made it all the way down here it is because your patience for physics, crappy pictures and bad jokes is... the bomb! I believe that, the development of the atomic model is a perfect example of how the scientific method works, with new proposals, advancing, being born from previous "errors", patching them up or upgrading them. From this evolution an atomic structure was born and thanks to it a "nucleus" was mentioned, starting a new branch of science: Nuclear Physics.
The development of this branch has an unavoidable context the WW2, that started a few months after the discovery of fission. HitIer's Germany had been preparing for this for quite a while. German-Jewish Scientists and any other people that that saw what was coming had a chance and migrated to other countries (Einstein fled back in 1932). Many of them are the ones that contributed to the development of the bomb (Enrico Fermi, for example). The development on the topic was a strategic point besides of scientific, many of the advances were achieved independently by the countries in war.
the physics of the small things went on strong, of course. Today electrons are the only that keep their status of "elemental particles" (And not even that under the String Theory). Today we know that protons and neutrons have internal structure, they are made by smaller particles: colored quarks.
So, there's parts within the subatomic particles that we found in that atom that we named literally, because it was "indivisible"... And we haven't updated the name!